
Did You Know?
- The current rate of change in ocean pH is roughly 50 times faster than known historical rates of change.
- Scientists estimate that the current rate of change in ocean pH has not likely occurred on the planet for the past 100 million years.
- More than one billion people worldwide get their primary protein food source from the ocean.
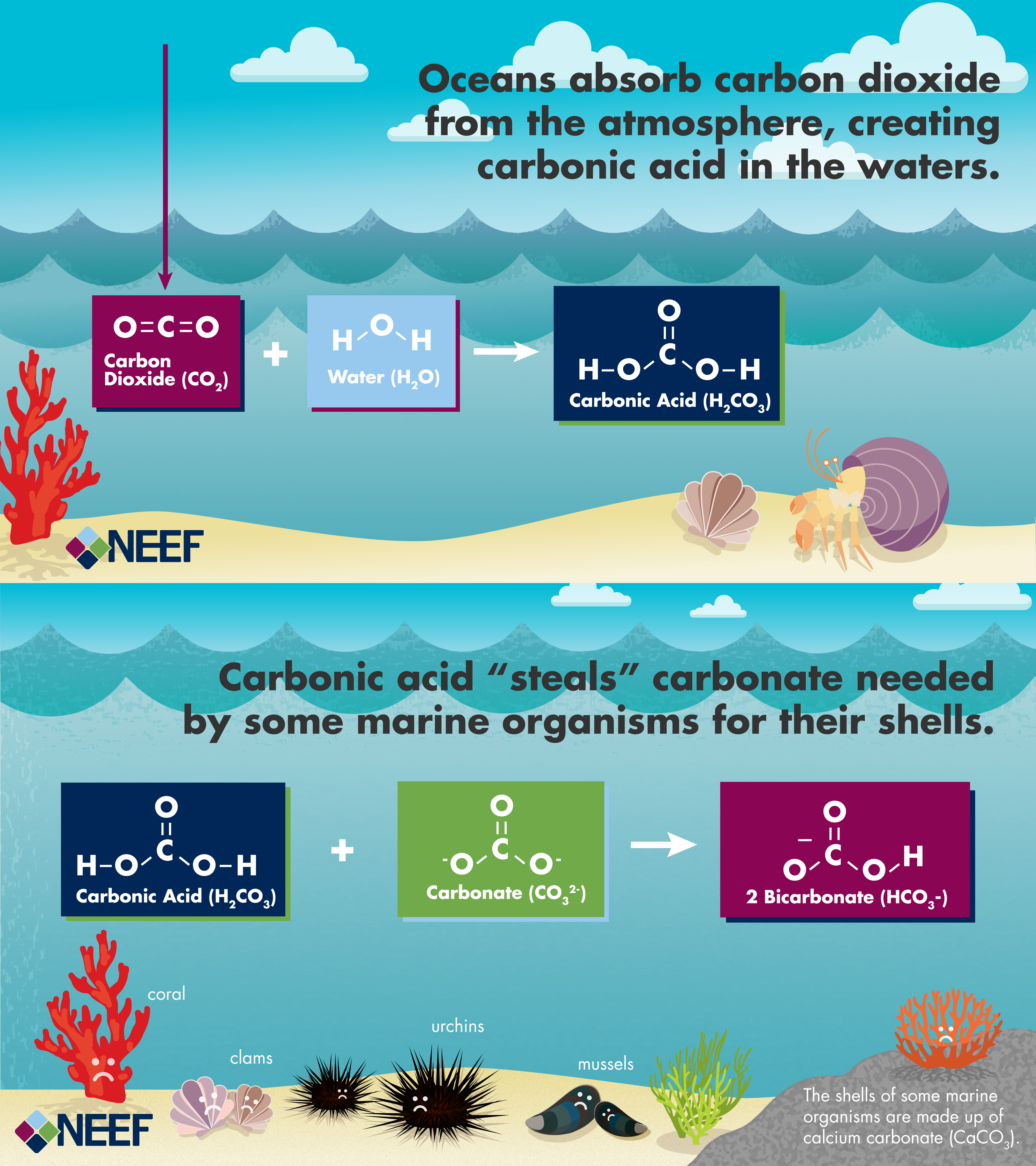
The amount of carbon dioxide (CO2) emitted into the atmosphere has risen about 40% above pre-industrial levels. The oceans absorb about 25% of CO2 released into the atmosphere, where the CO2 reacts with seawater to form carbonic acid, which lowers ocean pH levels, making the water more acidic.
The acidity of ocean surface waters has increased by about 30% over the last 250 years and could become nearly 150% more acidic by the end of the century if CO2 emission levels continue to increase at the current pace. Different regions of the ocean are more susceptible to an increase in acidification due to other factors such as coastal upwelling, river and glacial discharge, sea ice loss, and urbanization.
Increasing ocean acidity decreases the ability of shells and other calcium carbonate structures, such as coral skeletons, to form. Examples of sea life that are being directly affected are oysters, clams, sea urchins, shallow water corals, deep sea corals, and certain species of plankton. On a broader scale, the inability of these creatures to fully develop will also negatively impact other members of the food web that feed on them, including salmon, whales, and humans.
Learn More
- The illustration below demonstrates the chemical process of ocean acidification.
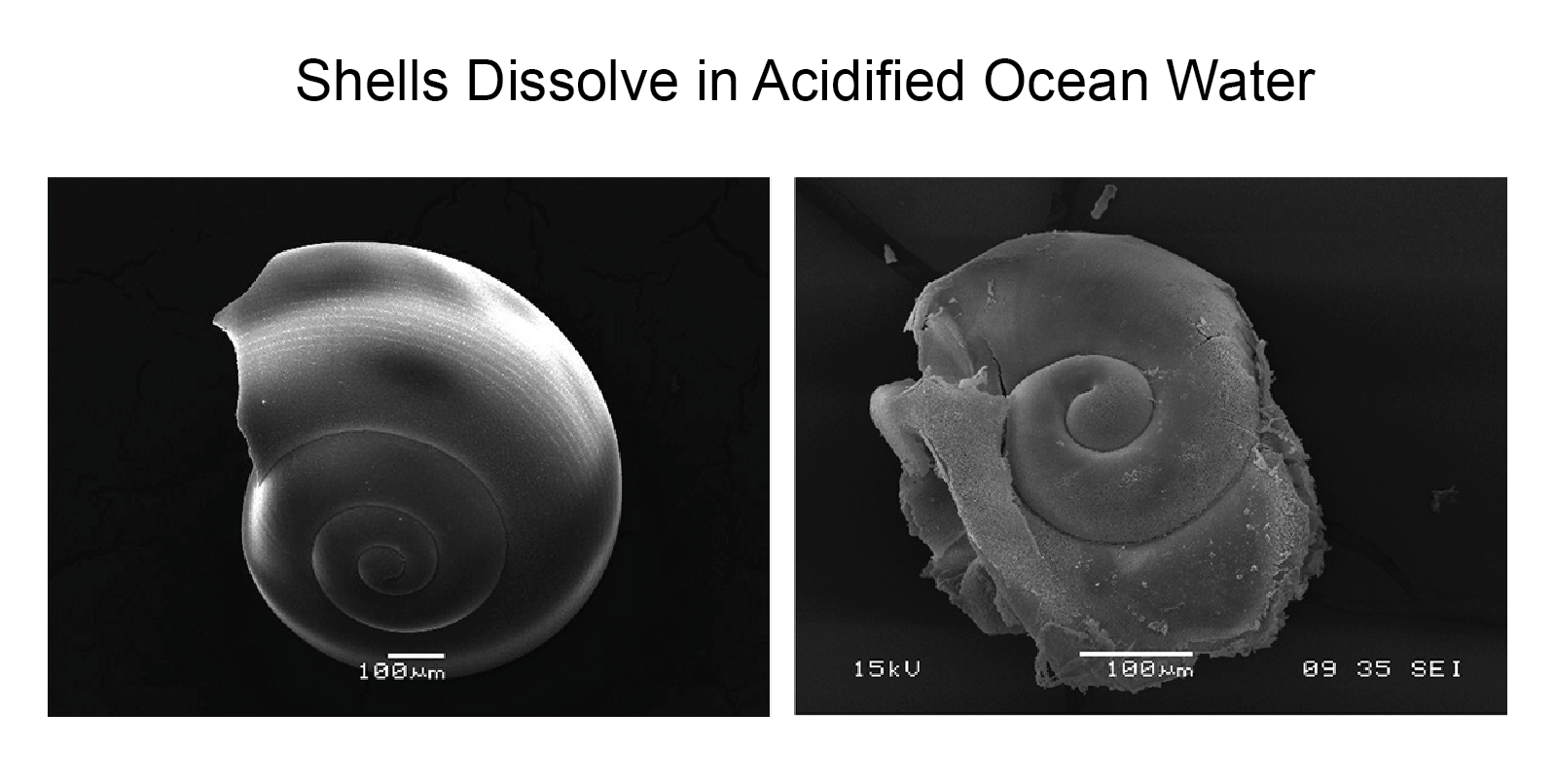
The photo below from the 2014 National Climate Assessment shows what happens to a pteropod's shell in seawater that is too acidic. On the left is a shell collected from a live pteropod from a region in the Southern Ocean where acidity is not too high. On the right is a shell from a pteropod collected in a region where the water is more acidic. Pteropods are small, free-swimming snails that are eaten by a range of marine species, from krill to whales. They are an important food source for North Pacific juvenile salmon.
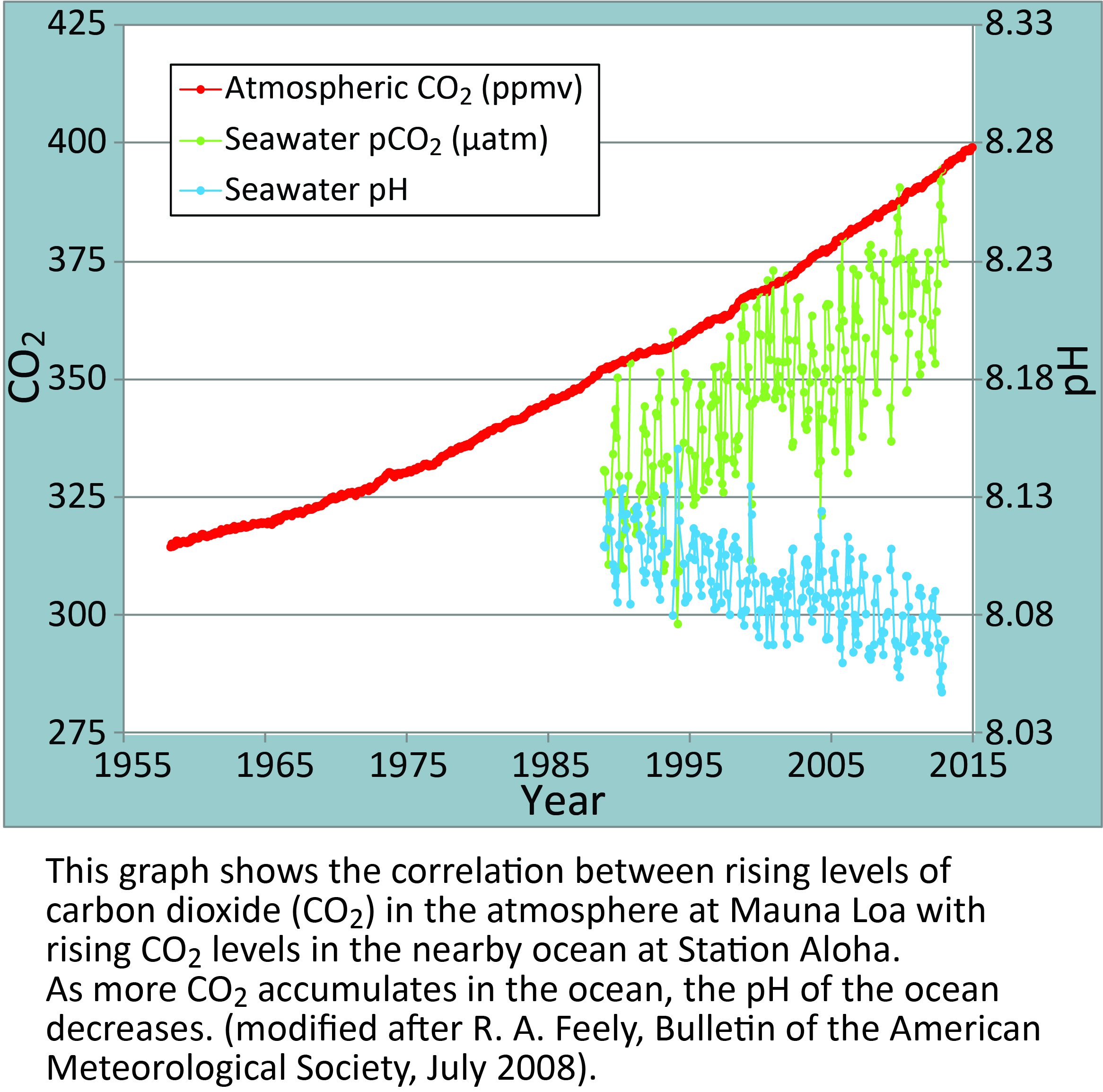
The graphic below from the NOAA PMEL Carbon Program shows how the seawater around Hawaii has become more acidic (decrease in pH) as levels of CO2 in the atmosphere and ocean have increased.
Sources:
- Melillo, Jerry M., Terese (T.C.) Richmond, and Gary W. Yohe, Eds. 2014. Climate Change Impacts in the United States: The Third National Climate Assessment. Washington: U.S. Global Change Research Program. http://nca2014.globalchange.gov/.
- NOAA. 2013. "Ocean Acidification." Accessed September 18, 2015. http://www.education.noaa.gov/Ocean_and_Coasts/Ocean_Acidification.html.
- NOAA. 2015. "Ocean Acidification: The Other Carbon Dioxide Problem." Accessed September 18. http://pmel.noaa.gov/co2/story/Ocean+Acidification.
- NOAA. 2015. "What is Ocean Acidification (OA)?" Accessed September 18. http://oceanacidification.noaa.gov/Home/WhatisOceanAcidification.aspx.
- US EPA. 2015. "Climate Change Indicators: Ocean Acidity." Accessed September 18. http://www.epa.gov/climatechange/science/indicators/oceans/acidity.html.
- US EPA. 2015. "Climate Students: Increased Ocean Acidity." Accessed September 18. http://www3.epa.gov/climatechange/kids/impacts/signs/acidity.html.
- The White House. 2014. The Challenge of Ocean Acidification. Washington: The White House Office of Science and Technology Policy. http://www.whitehouse.gov/sites/default/files/microsites/ostp/the_chall….


